Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Saito, Akito*; Sakuma, Hiroshi*; Oda, Chie; Honda, Akira; Sato, Tsutomu*
no journal, ,
Chemical transformations of montmorillonite, a key component of bentonite, by ammonium ion as a reducing product of nitrate, may influence the suitable physical properties like expandability of the bentonite as the buffer material in deep geological disposal system for nitrates containing radioactive waste. Basal spacing of ammonium-montmorillonite before or after the interaction with 1M-ammonia water (pH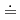 12) was studied by XRD. Molecular dynamics method was also used to simulate the behavior of cation and water in the interlayer of ammonium-montmorillonite. The experimental results suggested that the interlayer cation in montmorillonite did not change from ammonium ion even after the interaction with ammonia water (pH
12) was studied by XRD. Molecular dynamics method was also used to simulate the behavior of cation and water in the interlayer of ammonium-montmorillonite. The experimental results suggested that the interlayer cation in montmorillonite did not change from ammonium ion even after the interaction with ammonia water (pH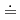 12) which ammonia(aq) was dominant species in, and crystalline swelling behavior of ammonium-montmorillonite differed from that of montmorillonite containing other type of interlayer cation. MD calculation indicated these features may be due to the hydrogen bond between ammonium ion and basal oxygen of montmorillonite.
12) which ammonia(aq) was dominant species in, and crystalline swelling behavior of ammonium-montmorillonite differed from that of montmorillonite containing other type of interlayer cation. MD calculation indicated these features may be due to the hydrogen bond between ammonium ion and basal oxygen of montmorillonite.
Saito, Akito*; Sakuma, Hiroshi*; Oda, Chie; Honda, Akira; Sato, Tsutomu*
no journal, ,
Adsorption of ammonium ion as a reducing product of nitrate on montmorillonite (Mt) may influence the suitable physical properties like expandability of the bentonite as the buffer material in geological disposal system for nitrates containing radioactive waste. Expandability and chemical stability of ammonium-Mt was studied by XRD measurement of the basal spacing and by molecular dynamics simulation. The experimental results and the simulations showed that (1) ammonium-Mt, unlike Mt with other types of interlayer cation, exhibited crystalline swelling with 1-layer hydrate structure under low relative humidity conditions, and this feature may be due to that mixing enthalpy in the ammonium-Mt and water system at low water content was negatively large compared with that of Mt with other types of interlayer cation, (2) the interlayer cation in Mt did not change from ammonium ion even after the interaction with ammonia water which ammonia (aq) was dominant species in, while (3) the interlayer ammonium ion was replaced by sodium ion by the interaction with sodium hydroxide solution.
Miyano, Riku*; Asanuma, Noriko*; Matsuura, Haruaki*; Aihara, Haruka; Watanabe, So; Nomura, Kazunori
no journal, ,
no abstracts in English
Aihara, Haruka; Watanabe, So; Nomura, Kazunori; Kamiya, Yuichi*
no journal, ,
no abstracts in English
Matsuura, Haruaki*; Kobayashi, Ami*; Miyoshi, Masahide*; Aihara, Haruka; Watanabe, So; Nomura, Kazunori
no journal, ,
no abstracts in English